Electron Paramagnetic Resonance
Preamble
The structure-activity relationship is one of the important aspects to have control over the
property of the targeted complexes and to fine-tune the structure of the complexes to realize
the improved activity in many research areas such in Chemistry, Biology, solid-state physics,
semi-conductors, display materials, catalysis, spin Tapping, etc. For example, to understand the
mechanism of a catalytic reaction (either a radical or non-radical pathway), nature of the
excited state involved in the various bio-mimetic and metalloenzyme oxidation reaction need
to be understood. The transient intermediates that are generated in many reactions, protein
folding, display devices (light or temperature or pressure-induced) are some of of the
cutting-edge research areas. Electron Paramagnetic Resonance (EPR) spectrometer helps to
study these intermediates and various associated processes
The instrument
EPR instrument is mainly used to characterize the paramagnetic complexes with unpaired
electrons and organic radicals. Unlike nuclear magnetic resonance (NMR), there is always an
element of hesitation and hurdle among the students to understand, analyze and interpret
the data that arises from the EPR, due to the complication arising from the g-anisotropy,
hyperfine interaction, and magnetic anisotropy in certain transition metal complexes such
as Fe(II). Therefore, we aim to introduce this sophisticated analysis at their preliminary level
to make the students realize the potential applications of these instruments besides teaching
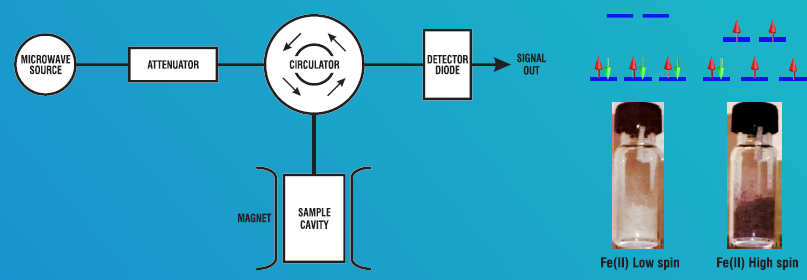
the basic principles, analysis, and interpretation of the EPR data. The generic instrument
consists of four major components 1) Microwave resonator 2) Microwave bridge 3) Magnet
system 4) Control electronics.
Experiments
Among the many experiments planned for UG/PG/Ph.D., students, one illustrative example
is the temperature-dependent spin state change of the Fe(II) complex i.e. temperature
assisted spin Crossover (SCO) of [Fe(NH2tra)3]Br2 (where NH2trz = 4-amino-1,2,4-triazole)
phenomenon. The SCO complexes are envisaged as molecular-based information storage
devices, molecular switches, and display materials, etc.,. At room temperature, the Fe(II)
complex exists as a high spin (S =2), paramagnetic while at low temperature (77 K) the same
complex exists as a low spin (S = 0), diamagnetic complex. EPR instrument is aimed at teaching
how to determine the electronic structure of both spin states from their EPR spectral
features, besides the fundamental theories of the instrument.

