Fast Reactions : Flash Photolysis :
Preamble
Many reactions in nature occur at an extremely fast time scale and are also complex. These
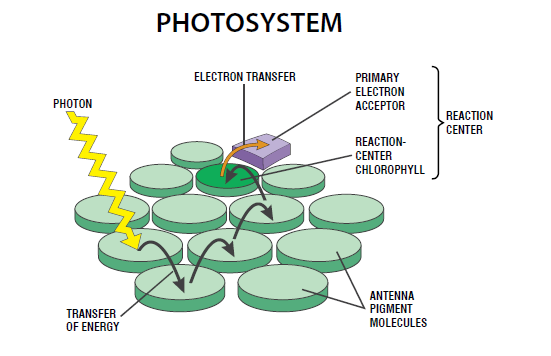
reaction are not resolvable in the time scale that humans can act. For instance, the light-harvesting
system of the photosynthetic reaction center, which is at the heart of the photosynthesis
process, occurs in the femtosecond time scale (a femtosecond is one-millionth of one-billionth
of a second). Similarly, the early chemical events in the process of vision occur in the picosecond
(one-millionth of one-millionth of a second) time scale. The time scale of early events in the
photosynthetic and vision process initiates a series of very complex processes, which ultimately
leads to an event that occurs on the time scale that humans can decipher without any aids. New
studies indicate that “the blink of an eye” could be as low as 15 milliseconds. Apart from these
examples, a vast number of reactions, either initiated or uninitiated by light, occur at timescales
that need special tools to investigate, to which this facility is geared.
The instrument
The timescale of a reaction is measured relative to a triggering event, which could be as
simple as adding two reacting chemicals. Alternatively, a large class of reactions occur due
to a “photo-trigger”, and are classfied as photochemical reactions. The initial events in a
photochemical reaction are known to cover a large time window at least of about ten
orders of magnitude (femtoseconds to microseconds) thus making it impractical for a
single instrument to cover this entire dynamic range. The LASER FLASH PHOTOLYSIS facility
is capable of probing light-induced events that occur on the time scale of nanoseconds
(one-billionth of a second) to microseconds (one-millionth of a second). A generic version
of the instrument consists of three major components, which are (i) photolysis laser, (ii)
probe unit and (iii) detection unit. The three units are in turn connected by a master
controller which also acts as a time-keeper.
Experiments
One illustrative example, among many experiments that are planned for UG/PG/PhD
students, is the photo-redox reaction of quinone coupled with proton transfer. In this
reaction quinone upon excitaion with light is converted to hydroquinone through an
intermediate semiquinone. This is also a classical example of consecutive reactions, wherein
the concentration of the intermediate semiquinone builds up in the first half and decays
in the second half. This facility is aimed to teach and train students to understand the
concepts in light-induced reactions.

